Key Points
- Glass exhibits complex physics with molecules in disordered, frozen states. Supercooled liquids lack long-range order yet are not fully liquid.
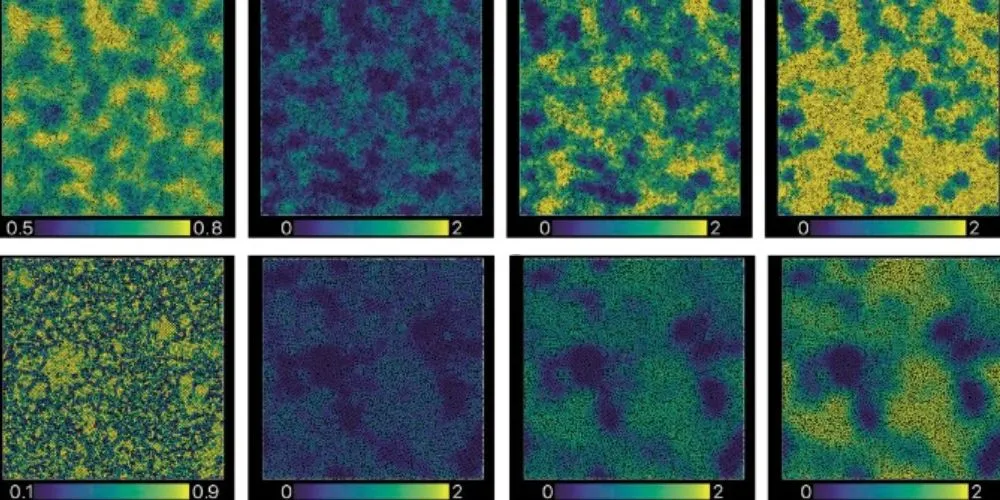
- The study linked microscopic structural order to dynamic behavior.
- The T1 process determines Arrhenius or super-Arrhenius behavior in glass dynamics.
- Findings may lead to stronger, more efficient glass manufacturing.
Glass, an everyday material, harbors intricate and poorly understood physics. Despite its appearance of simplicity, the molecular structure of glass remains a subject of fascination for scientists. Medieval stained-glass windows, for example, have maintained their rigidity for centuries due to molecules locked in a state of perpetual disorder. Similarly, supercooled liquids blur the line between solid and liquid, with their particles lacking both long-range lattice order and sufficient energy for free movement.
In a groundbreaking study published in Nature Materials, researchers from the Institute of Industrial Science at the University of Tokyo used advanced computer simulations to unravel the microscopic dynamics of supercooled liquids. The research focused on Arrhenius activation energy, the energy barrier required for a process to proceed.
In disordered materials, rearranging individual particles demands energy, with rates decreasing exponentially as the energy barrier increases. This phenomenon, known as “Arrhenius behavior,” becomes even rarer at low temperatures when particle rearrangements require cooperative motion, giving rise to “super-Arrhenius” relationships.
The study was the first to connect structural order and dynamic behavior in liquids at a microscopic level. Using numerical analysis, the researchers identified a process termed “T1” as central to understanding the cooperative dynamics of glass-forming liquids. If the T1 process disrupts local structural order, it produces Arrhenius-like behavior through independent particle motion. Conversely, if T1 maintains local order cooperatively, it propagates dynamic influence outward, leading to super-Arrhenius behavior.
Lead author Seiichiro Ishino emphasized the importance of these findings in explaining the origins of dynamic cooperativity in glass-forming substances. Senior author Hajime Tanaka highlighted their potential for advancing material design and manufacturing processes, paving the way for stronger and more durable glass products like those used in smartphones.