Key Points
- The University of Münster team developed a new method for creating stable 3D cage molecules.
- These cage molecules offer more stability than conventional flat aromatic rings used in pharmaceuticals.
- The breakthrough involves inserting nitrogen, oxygen, and carbon atoms into highly reactive bicyclobutane rings using light energy.
- The 3D rings could be alternatives to flat heteroaromatic rings commonly found in drug molecules, enhancing stability and effectiveness.
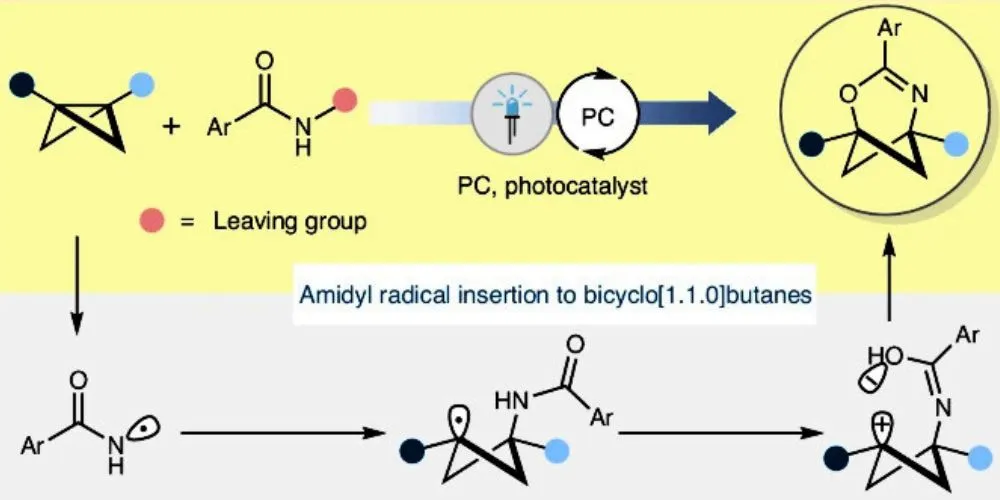
A research team at the University of Münster, led by chemist Frank Glorius, has developed a novel method for creating ring-shaped “cage molecules” that could revolutionize drug development. Unlike conventional flat molecular rings, these three-dimensional structures offer increased stability, making them promising alternatives for pharmaceutical applications. The team’s findings were published in the journal Nature Catalysis, highlighting the potential of these innovative cage molecules to replace less stable flat aromatic rings commonly found in drugs.
Aromatic rings, which are flat and found in organic molecules, are frequently used in pharmaceuticals and agrochemicals. However, their flat structure can be unstable under physiological conditions, reducing the effectiveness of drugs. Scientists have long sought more stable, three-dimensional alternatives to these rings. While substitutes for simple flat rings like benzene (a six-carbon atom ring) have been developed, synthesizing 3D versions of rings containing other essential atoms such as nitrogen, oxygen, or sulfur—known as “heteroaromatic” rings—has proven to be much more difficult.
The Münster team achieved their breakthrough by using bicyclobutane, a highly reactive molecule, and a light-sensitive catalyst to trigger the chemical reaction. By employing light energy, they could insert nitrogen, oxygen, and carbon atoms into this reactive bicyclic molecule, creating a new type of 3D ring. By incorporating heteroatoms like nitrogen and oxygen, the team could create more complex, cage-like 3D rings, which could serve as stable alternatives to flat heteroaromatic rings in drug molecules.
“This is a significant development,” said Prof. Frank Glorius, “as the new rings offer a more stable structure that could potentially enhance drug efficacy.” Dr. Chetan Chintawar added, “These 3D molecules are versatile, stable, and can be easily modified, making them ideal for the creation of various other cyclic molecules.”
The team conducted both experimental and computational studies to understand the reaction mechanism. They suggest the process begins with a light-induced electron transfer from the excited catalyst to the reactants, forming the final 3D products. This discovery opens up new possibilities in drug development, particularly in creating more effective and stable medications.