Key Points
- University of Leoben researchers developed a new method for hydrogen storage using reactive polymers.
- The process involves catalytic hydrogenation and dehydrogenation reactions in polymers like polyvinyl naphthalene.
- This method stores around 5% by weight of hydrogen without requiring high pressures or low temperatures.
- The polymer-based approach offers enhanced safety, cost-efficiency, and ease of handling compared to traditional storage methods.
Scientists at the University of Leoben have made a significant breakthrough in safely storing hydrogen, a key challenge in clean energy. The research team has developed an innovative method using reactive polymers to store hydrogen chemically. This discovery could have far-reaching implications, particularly in mobility and decentralized hydrogen supply, offering a safer and more practical approach to hydrogen storage.
Hydrogen is often hailed as an efficient and clean energy carrier, with potential applications ranging from fuel for vehicles and heating systems to large-scale energy storage. However, traditional methods of storing hydrogen face several challenges, such as in high-pressure tanks or cryogenic liquids, face safety, cost, and efficiency. These methods often require low temperatures or high pressures, making them expensive and complex. The new polymer-based storage system offers a promising alternative.
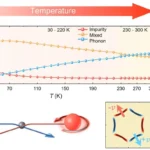
The Leoben team, consisting of researchers Mohammadhossein Sharifian, Wolfgang Kern (recently deceased), Gisbert Riess, and Nikolaos Kostoglou, discovered that certain polymers can chemically bind hydrogen and release it safely.
The process involves catalytic hydrogenation and dehydrogenation reactions within reactive polymers, such as polyvinyl naphthalene. This method allows the storage of approximately 5% of hydrogen by weight in polymers without the need for low temperatures or high pressures. According to Professor Riess, this process offers several advantages over traditional storage methods, including enhanced safety, cost-effectiveness, and ease of handling.
Unlike metal hydrides or liquid organic carriers commonly used in hydrogen storage, the polymer-based method is safer due to its stability. The hydrogen is chemically bonded in the polymer, remaining securely stored until needed. This eliminates the risks associated with high-pressure hydrogen storage and makes transporting and handling the hydrogen easier.
The researchers believe this storage method’s efficiency and capacity can be improved further with further refinement and optimization. Their ongoing research focuses on various vinyl aromatic polymers that can store or release hydrogen under different conditions, aiming to make hydrogen storage more accessible and practical for widespread use.
The research team’s findings, published in the International Journal of Hydrogen Energy, mark an important step toward making hydrogen a more viable and sustainable energy solution. This breakthrough could pave the way for safer, more cost-effective hydrogen storage solutions, driving the adoption of hydrogen as a clean energy source.