Researchers from the Medical Research Council (MRC) Toxicology Unit at the University of Cambridge have made a significant breakthrough in enhancing the safety of mRNA (messenger ribonucleic acid) therapeutics. The research, published in the journal Nature, reveals that misreading therapeutic mRNAs by the cell’s decoding machinery can trigger unintended immune responses in the body.
The team, led by biochemist Professor Anne Willis and immunologist Dr James Thaventhiran, identified a specific mRNA sequence responsible for these off-target effects and developed a solution to prevent them. This research was funded by the Medical Research Council and the Wellcome LEAP R3 program, with support from the NIHR Cambridge BRC.
mRNA serves as genetic material instructing cells to produce specific proteins, making it a key component in revolutionary therapeutics, including COVID-19 vaccines. The decoding machinery, known as ribosomes, sometimes “slips” when faced with repeats of a chemical modification found in mRNA therapeutics. This results in the production of off-target proteins, leading to unintended immune responses.
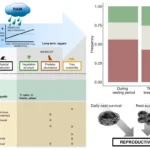
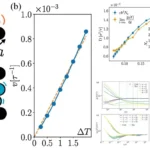
The researchers focused on bases with a chemical modification called N1-methyl pseudouridine, commonly present in mRNA therapies, as the cause of these slips. Collaboration with other universities allowed them to test for evidence of off-target protein production in individuals who received the mRNA Pfizer vaccine against COVID-19. While an unintended immune response occurred in one-third of the participants, no adverse effects were observed.
The team then redesigned mRNA sequences, correcting error-prone genetic sequences in synthetic mRNA to avoid off-target effects. This modification ensured the production of the intended proteins, offering a preventive measure against hazardous immune responses in future mRNA-based therapeutics.
Dr James Thaventhiran emphasized the safety of mRNA vaccination against COVID-19 and stressed the importance of ensuring the reliability of future mRNA medicines. Professor Anne Willis highlighted the potential of mRNA therapeutics for treating various diseases, emphasizing the need for safety to avoid unintended side effects.
The research contributes to the rapid implementation of measures to prevent safety issues associated with mRNA therapeutics, ensuring their safety and efficacy. Using synthetic mRNA for therapeutic purposes is cost-effective and holds promise for addressing global health inequalities. The findings have implications for the ongoing development of mRNA treatments, reinforcing their safety profile.