In the long and arduous war against cancer, medicine has historically relied on the “Big Three”: surgery (cut it out), chemotherapy (poison it), and radiation (burn it). While these treatments have saved millions of lives, they are blunt instruments. They often damage healthy tissue alongside the cancer, leading to debilitating side effects, and for some patients, they simply stop working.
For decades, scientists dreamed of a fourth pillar of cancer care—one that is precise, personalized, and persistent. This dream is now a reality. It is called CAR-T Cell Therapy.
Short for Chimeric Antigen Receptor T-Cell Therapy, this revolutionary treatment involves genetically engineering a patient’s own immune cells to hunt down and destroy cancer. It is not just a drug; it is a “living drug.” Since its first FDA approval in 2017, CAR-T has transformed the prognosis for patients with certain blood cancers, particularly leukemia, offering the possibility of a cure where previously there was none.
This comprehensive guide explores the science behind CAR-T, the patient journey, the success stories, the risks, and the future of this groundbreaking immunotherapy.
The Science: Engineering a Super-Soldier
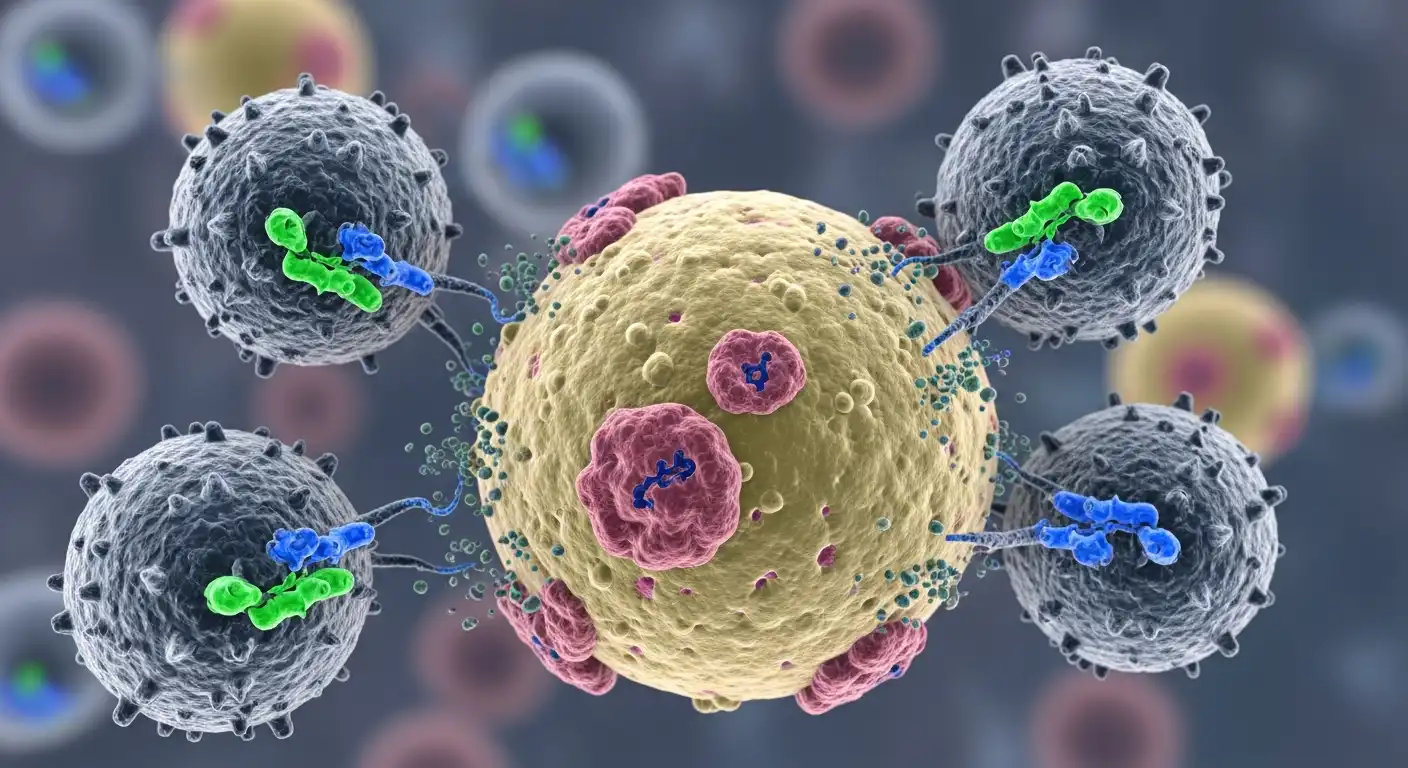
To understand CAR-T, we must first look at the human immune system. Our bodies are constantly patrolling for threats. T-cells are the “special forces” of this system. They are designed to identify infected or abnormal cells and kill them.
However, cancer is tricky. Cancer cells are the body’s own cells gone rogue. They often wear “camouflage” or develop mechanisms to shut down T-cells, allowing the tumor to grow unchecked. CAR-T therapy strips away this camouflage.
The Process: From Vein to Lab and Back
The creation of CAR-T cells is a feat of modern biotechnology involving three main steps:
- Leukapheresis (Collection): Blood is drawn from the patient. A machine separates the white blood cells (which contain T-cells) and returns the red blood cells and plasma to the body.
- Genetic Engineering (The Upgrade): In a specialized laboratory, scientists introduce a new gene into the T-cells using a deactivated virus. This gene instructs the T-cell to grow a new receptor on its surface—the Chimeric Antigen Receptor (CAR).
- Think of the CAR as a synthetic GPS tracker. It is designed to bind specifically to a protein (antigen) found on the surface of the cancer cell. For leukemia (specifically B-cell Acute Lymphoblastic Leukemia), the target is usually the CD19 antigen.
- Expansion and Infusion: The newly engineered CAR-T cells are grown in the lab until they number in the millions. They are then frozen and shipped back to the hospital, where they are infused into the patient’s bloodstream.
Once inside the body, these super-charged T-cells act like heat-seeking missiles. They latch onto the cancer cells and release toxic chemicals that destroy them. Furthermore, because they are living cells, they multiply in the body, creating an army that can persist for years, providing long-term surveillance against relapse.
The Success Stories: “The Emily Whitehead Effect”
The potential of CAR-T therapy is perhaps best illustrated by the story of Emily Whitehead. In 2012, at age 6, Emily was battling relapsed Acute Lymphoblastic Leukemia (ALL). She had exhausted all standard treatments and was given weeks to live.
She became the first child to receive an experimental CAR-T therapy (now known as Kymriah) at the Children’s Hospital of Philadelphia. After a severe immune reaction that nearly killed her, she woke up cancer-free. Over a decade later, Emily remains cancer-free. Her story ignited global interest and investment in the field, proving that terminal cancer could be reversible.
Clinical Data and Approvals
Since then, the FDA has approved multiple CAR-T therapies, including:
- Kymriah (Tisagenlecleucel): For pediatric and young adult ALL, and certain lymphomas.
- Yescarta (Axicabtagene ciloleucel): For large B-cell lymphoma.
- Tecartus: For Mantle Cell Lymphoma.
In clinical trials for relapsed B-cell ALL—patients who had no other options—remission rates have reached as high as 80% to 90%. While not every remission is permanent, these numbers are unprecedented in the history of oncology for such advanced disease stages.
The Risks: Managing the Cytokine Storm
CAR-T is powerful, and with great power comes significant risk. The treatment triggers a massive immune response that can be life-threatening if not managed by expert teams.
Cytokine Release Syndrome (CRS)
When CAR-T cells attack cancer, they release massive amounts of inflammatory proteins called cytokines. This is known as a “Cytokine Storm.” Symptoms range from high fevers and fatigue to dangerous drops in blood pressure and organ failure.
- Management: Ironically, the drug used to save Emily Whitehead (Tocilizumab) is an arthritis medication that blocks a specific cytokine (IL-6). It is now the standard antidote for severe CRS, allowing doctors to calm the storm without stopping the cancer fight.
Neurotoxicity (ICANS)
Some patients experience neurological side effects, known as ICANS (Immune Effector Cell-Associated Neurotoxicity Syndrome). Symptoms can include confusion, difficulty speaking (aphasia), seizures, or even swelling of the brain. While terrifying, these symptoms are usually temporary and resolve within a few weeks.
B-Cell Aplasia
Because the therapy targets the CD19 protein found on all B-cells (both cancerous and healthy), it destroys the patient’s healthy B-cells too. B-cells are responsible for making antibodies. Patients often require monthly infusions of immunoglobulins (IVIG) to protect them from infections indefinitely.
The Cost and Access Challenge
One of the biggest hurdles for CAR-T is not biological, but economic. These are “bespoke” treatments. Making a personalized batch of cells for one specific patient is incredibly expensive.
- The Price Tag: A single infusion can cost between $375,000 and $475,000, not including hospital stays and supportive care. The total cost of care can exceed $1 million.
- Manufacturing Time: It takes 2-4 weeks to manufacture the cells. For a patient with rapidly progressing leukemia, this wait time can be fatal.
Researchers are working on “Off-the-Shelf” (Allogeneic) CAR-T cells. These are cells derived from healthy donors that are genetically edited to be safe for any patient. This would allow hospitals to keep doses in the freezer, ready for immediate use, and drastically lower the cost.
The Future: Beyond Blood Cancers
Currently, CAR-T is only FDA-approved for “liquid” cancers (leukemias, lymphomas, myeloma). The “Holy Grail” is treating Solid Tumors (breast, lung, and colon cancer).
Solid tumors are harder targets. They have a hostile microenvironment that suppresses immune cells, and they are harder for the T-cells to penetrate. Furthermore, finding an antigen that is present only on the tumor and not on healthy organs (to avoid autoimmune damage) is difficult. Scientists are engineering “Armored CARs” that secrete proteins to break down the tumor’s defenses, and multi-target CARs to prevent the cancer from escaping.
Conclusion
CAR-T Cell Therapy represents a paradigm shift in medicine. We are moving away from chemical compounds and radiation beams toward a future where cells are the medicine. For leukemia patients who have run out of options, CAR-T offers something more valuable than just another treatment: it offers time, and the genuine possibility of a cure.
While challenges remain—cost, toxicity, and expansion to solid tumors—the trajectory is clear. The immune system is the ultimate weapon against cancer, and we have finally learned how to aim it.