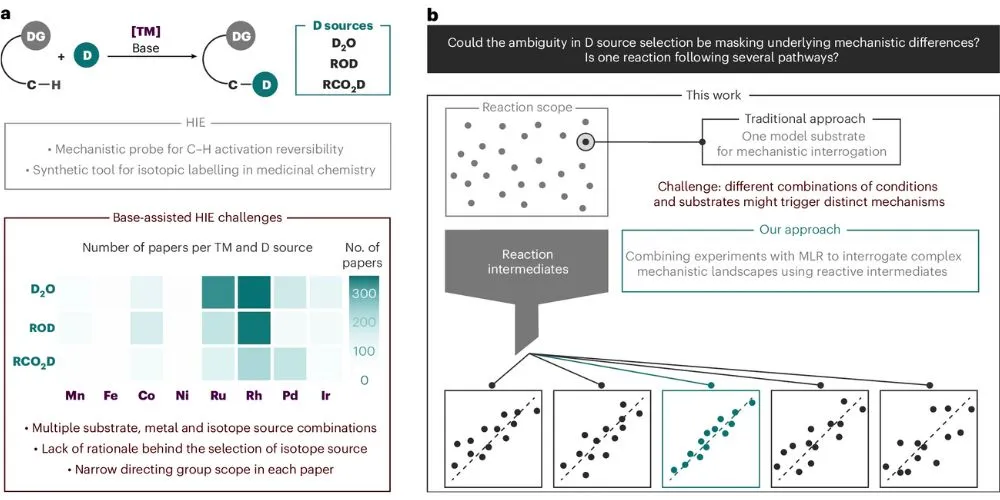
Key Points
- The choice of deuterium source changes the reaction pathway.
- Researchers combined lab work with data science for results.
- The findings are crucial for developing new deuterated drugs.
- A single catalyst acted in three different ways under different conditions.
A new collaboration has revealed that making slight changes to chemical ingredients does much more than just tweak the final result—it can completely change how a reaction happens. Published in Nature Catalysis, researchers from the Institute of Chemical Research of Catalonia in Spain and Ben-Gurion University in Israel found that specific additives can force a chemical reaction to take entirely different paths.
The team focused on a process called hydrogen isotope exchange. This technique allows scientists to swap hydrogen atoms in a molecule with deuterium, a heavier version of hydrogen. This is a vital tool in the pharmaceutical industry.
Drugs made with deuterium often last longer in the body or have fewer side effects, and the FDA has already approved medicines using this technology. However, until now, picking the right source of deuterium—whether heavy water or an acidic chemical—has often been a guessing game.
By combining lab experiments with computer data analysis, the researchers found something surprising. They realized that the liquid used to provide the deuterium acts like a traffic cop. It directs the reaction down different roads based on the specific structure of the molecule they are working with.
For instance, using the same cobalt catalyst, the reaction shifted between three different mechanisms just by changing the conditions. Intuition alone could not predict these shifts.
Professor Mónica H. Pérez-Temprano explains that scientists can no longer assume a reaction works the same way for every chemical. This challenges the traditional approach of studying a single “model” reaction and assuming it applies to everything.
Professor Anat Milo added that this new data-driven approach helps provide a clearer picture. Instead of looking at a single reaction in isolation, they can now see how behaviors change across a wide range of conditions. This discovery gives chemists better tools to control how they build molecules, potentially leading to smarter and more efficient drug development in the future.