Key points
- University of Delaware researchers developed a new catalyst for plastic upcycling.
- The catalyst uses mesoporous MXenes to enhance the conversion of plastic waste into liquid fuels.
- Reaction rates are nearly double those of previous methods for LDPE hydrogenolysis.
- The catalyst exhibits high selectivity, minimizing the formation of unwanted byproducts, such as methane.
A team of researchers at the University of Delaware has made a significant breakthrough in tackling plastic pollution. They’ve developed a novel catalyst that dramatically accelerates the conversion of plastic waste into valuable liquid fuels. This innovative approach offers a faster, more efficient, and environmentally friendly solution compared to traditional recycling methods, which often degrade the plastic’s quality with each cycle and struggle to keep pace with the ever-growing volume of global plastic waste.
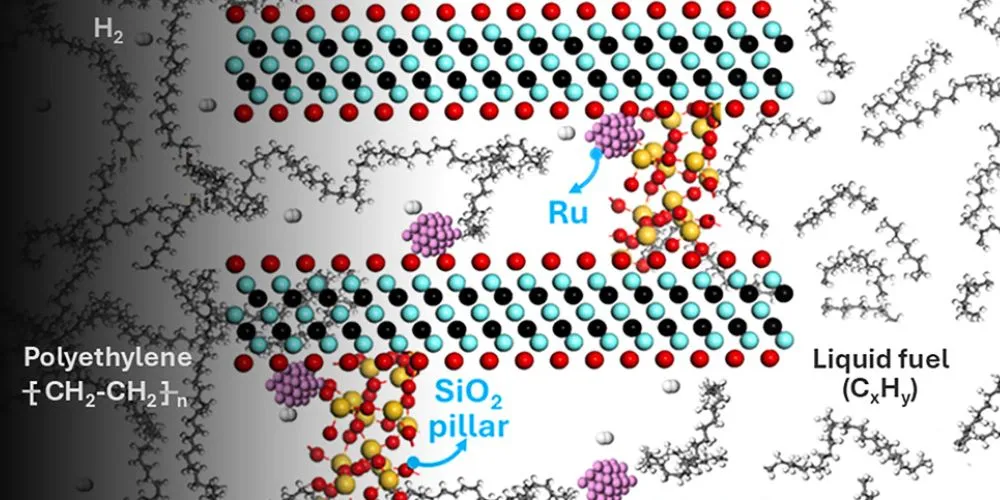
The research, featured on the cover of Chem Catalysis, focuses on hydrogenolysis, a process that uses hydrogen gas and a catalyst to break down polymers in plastics into usable liquid fuels.
The key to the team’s success lies in the use of mesoporous MXenes, a type of nanomaterial with larger, more open pores than previously utilized catalysts. These mesoporous MXenes, characterized by a structure similar to the pages of an open book, facilitate much easier interaction between the plastic polymers and the catalyst’s active sites.
This enhanced accessibility significantly speeds up the reaction. The researchers incorporated silica pillars to increase the porosity of the MXenes further, thereby improving the flow of both the plastic and the intermediate compounds formed during the reaction.
Testing the new catalyst with low-density polyethylene (LDPE), a common plastic found in shopping bags and films, yielded impressive results. The reaction rate was almost twice as fast as that reported in previous studies on LDPE hydrogenolysis.
Furthermore, the catalyst displayed remarkable selectivity, maximizing the production of liquid fuels while minimizing undesirable byproducts, such as methane, a potent greenhouse gas. This high selectivity is attributed to the stabilization of ruthenium nanoparticles within the mesoporous structure of the MXenes.
The researchers believe this advancement has the potential to revolutionize plastic waste management. They plan to refine the catalyst further and expand its application to various types of plastics. Their ultimate goal is to collaborate with industry partners to transform plastic waste from an environmental burden into a valuable resource, creating both economic opportunities and a more sustainable future.