Key Points:
- Polymer micelles act as tiny vehicles to transport medicine in the body.
- Previous research focused on water rather than salty bodily fluids.
- Chiba University used X-rays to study how these particles act in saline.
- Salt causes the particles to bind tighter but break down faster.
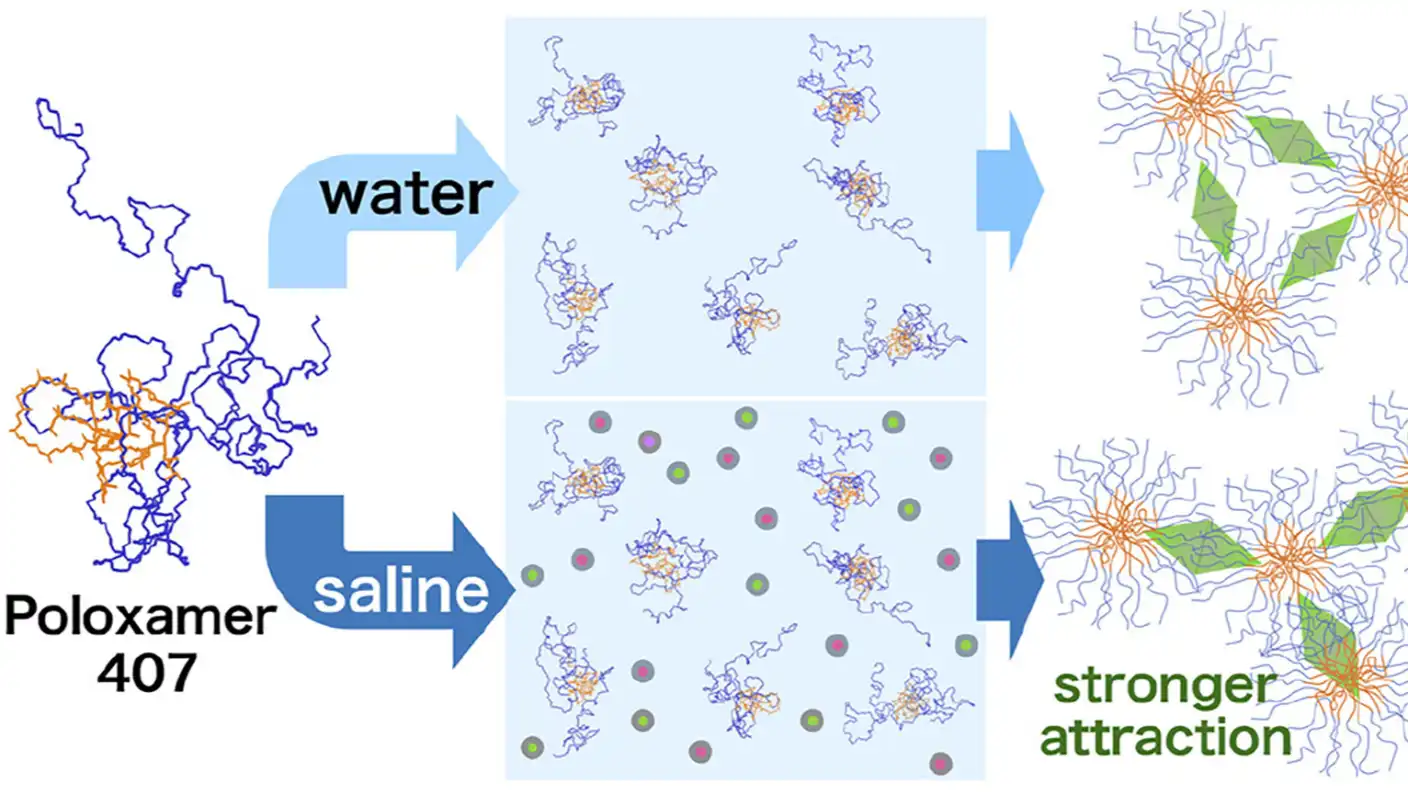
Tiny particles called polymer micelles are changing how doctors treat diseases. These microscopic spheres act like cargo ships, trapping drugs that are hard to dissolve and carrying them safely through the body. A specific polymer called Poloxamer 407 (P407) is famous for turning into a gel as it warms up to body temperature, which allows it to release medicine slowly over time.
However, there was a gap in what scientists knew. Most past research on P407 happened in pure water. The human body is not pure water; it is full of salts and ions. Without studying these particles in a salty environment, researchers could not be 100% sure how they would react inside a real patient.
A team led by Associate Professor Takeshi Morita at Chiba University in Japan decided to fix this. Instead of relying on old theories, they observed how P407 behaves in a saline solution that mimics actual bodily fluids. They used advanced X-ray scattering and laser technology to measure the size and movement of the particles.
The team discovered that salt makes a big difference. In the saline solution, the micelles attracted each other much more strongly than they did in plain water. They connected tightly, but this strong attraction actually caused the gel structure to be less uniform.
This behavior directly affects how stable the medicine remains. The researchers found that gels formed in saline broke down at lower temperatures than those in water. This means the structural fluctuations caused by the salt make the gel collapse sooner as it heats up.
Understanding this “clumping” behavior is vital for the future of medicine. Many modern drugs, including cancer treatments and anti-inflammatories, rely on these carriers. If scientists can predict how the carrier acts in salty body fluids, they can design more effective treatments.
Dr. Morita believes this work is a major step forward. By clarifying these physical interactions, the study paves the way for medicines that work better and are less of a burden on patients.
Source: Journal of Colloid and Interface Science (2026).