Key Points
- A novel two-stage carboplatin delivery system inhibits tumor growth and enhances radiotherapy.
- Hydrogel releases carboplatin early, while carboplatin particles target cancer cells later.
- Preclinical trials showed tumor eradication in mice with minimal radiation doses.
- The study demonstrates the potential for clinical impact and multidisciplinary innovation in glioma treatment.
Malignant gliomas, particularly glioblastoma and anaplastic astrocytoma, represent over 70% of adult primary brain cancers. Despite aggressive treatments like tumor excision and concurrent chemoradiotherapy, local recurrence remains a significant challenge, limiting survival outcomes.
Key obstacles include tumor proliferation during the interval between surgery and radiotherapy and the tumor’s inherent resistance to radiation combined with the brain’s limited tolerance for high radiation doses. This creates two critical clinical questions: how to suppress tumor growth during the post-surgical interval and amplify radiotherapy’s effects while minimizing radiation exposure.
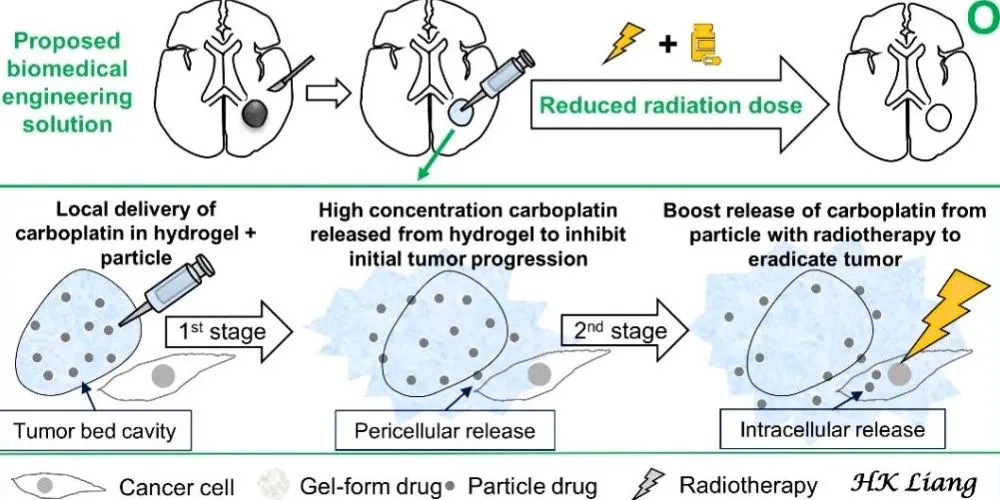
To address these challenges, researchers have developed an innovative local drug delivery system that enhances the efficacy of radiotherapy by utilizing carboplatin, an anticancer agent, and radiosensitizer. The research published in Biomaterials, the study is set for the January 2025 issue and is already accessible online.
This breakthrough system employs a two-stage controlled-release mechanism. Initially, a hydrogel releases high concentrations of carboplatin into the tumor’s pericellular space to inhibit tumor growth before radiotherapy. Once the hydrogel degrades, embedded carboplatin particles are internalized by cancer cells. Inside the cells, these particles degrade in the acidic environment of endosome–lysosome complexes, releasing concentrated carboplatin to intensify the anticancer effects of subsequent radiotherapy.
This dual-stage delivery system has demonstrated remarkable success in preclinical trials. It effectively suppresses initial tumor proliferation and works synergistically with low-dose radiotherapy to eradicate malignant gliomas in mice. The precise timing and localized release of carboplatin enhance radiotherapy outcomes and minimize the risk of damaging surrounding healthy brain tissue.
Developed through a collaboration between the National Taiwan University’s Department of Biomedical Engineering and the Division of Radiation Oncology at the National Taiwan University Hospital, this research highlights the power of multidisciplinary approaches in tackling complex clinical problems.
Dr. Liang, a radiation oncologist and neurologist involved in the study, emphasized the mission of improving treatment efficacy and patient quality of life. He also highlighted the commitment to fostering multidisciplinary teams to advance brain tumor therapies while reducing side effects.