Key Points:
- Rice University engineers develop the smallest implantable brain stimulator, the Digitally programmable Over-brain Therapeutic (DOT).
- DOT utilizes magnetoelectric power transfer technology to stimulate the brain wirelessly, offering greater patient autonomy and accessibility.
- Successful tests in human patients and pigs demonstrate the device’s efficacy in stimulating the motor cortex and ensuring stability.
- Motif Neurotech is seeking FDA approval for long-term clinical trials in humans, marking a significant milestone in neurostimulation-based therapies.
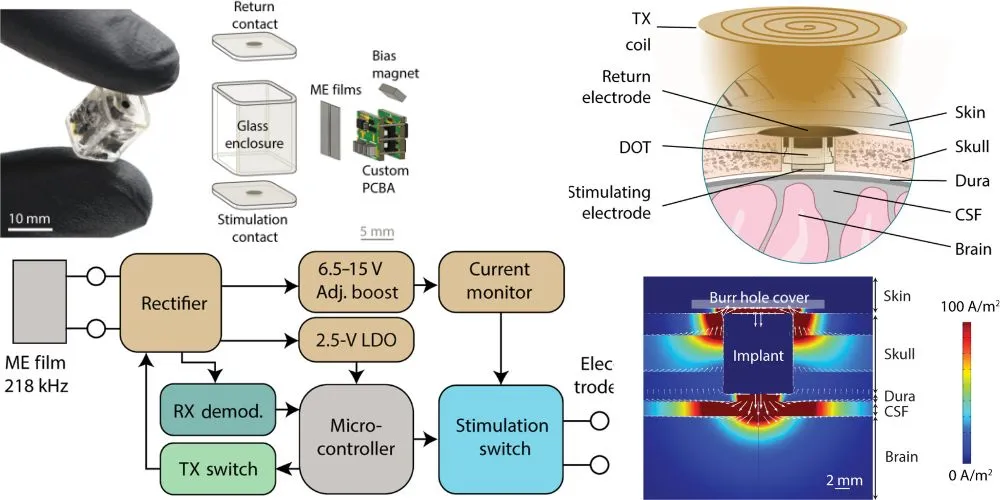
Rice University engineers have achieved a groundbreaking feat by developing the smallest implantable brain stimulator demonstrated in a human patient. This remarkable device, dubbed the Digitally programmable Over-brain Therapeutic (DOT), holds the potential to revolutionize the treatment landscape for drug-resistant depression and other neurological disorders.
“We eliminated the need for a battery by wirelessly powering the device using an external transmitter,” explained Joshua Woods, an electrical engineering graduate student in the Robinson lab and lead author on the study published in Science Advances.
Powered wirelessly via an external transmitter, the DOT offers a therapeutic alternative that prioritizes patient autonomy and accessibility while minimizing invasiveness. Developed in collaboration with Motif Neurotech and clinicians Dr. Sameer Sheth and Dr. Sunil Sheth, the pea-sized device stimulates the brain through the dura, the protective membrane attached to the bottom of the skull.
Existing implantable technologies for brain stimulation rely on large batteries and long wires, posing significant surgical risks and hardware burdens. The DOT eliminates the need for batteries, utilizing pioneering magnetoelectric power transfer technology to convert magnetic fields into electrical pulses efficiently. With a width of only 9 millimeters, the device delivers 14.5 volts of stimulation, marking a significant advancement in wireless power transfer technologies.
The researchers successfully tested the device in a human patient, demonstrating its ability to stimulate the motor cortex and evoke hand movement responses. Additionally, stability tests conducted in pigs showed promising results, validating the device’s efficacy over a 30-day duration.
The potential applications of the DOT extend beyond motor cortex stimulation, with prospects including targeting other brain regions to enhance executive functioning in individuals with depression or other disorders. Moreover, the device’s compact size and wireless functionality pave the way for convenient at-home usage, empowering patients to manage their treatment independently.
Implantation of the DOT requires a minimally invasive 30-minute procedure, resulting in virtually invisible incisions and same-day discharge. Compared to traditional deep brain stimulation (DBS), which entails more invasive procedures and higher perceived risks, the DOT offers a safer and more tolerable alternative.
Motif Neurotech is actively seeking FDA approval for long-term human clinical trials in collaboration with researchers and clinicians. This milestone marks a significant step forward in neurostimulation-based therapies, promising adaptive and personalized treatments based on individual brain signatures.