Key Points
- Scientists have a new theory that explains why snowflakes form in a variety of shapes.
- The theory is based on a tiny, invisible layer of liquid water on the surface of ice.
- The thickness of this “premelting film” changes with temperature and pressure.
- These changes cause the different faces of the ice crystal to grow at different rates, creating different shapes.
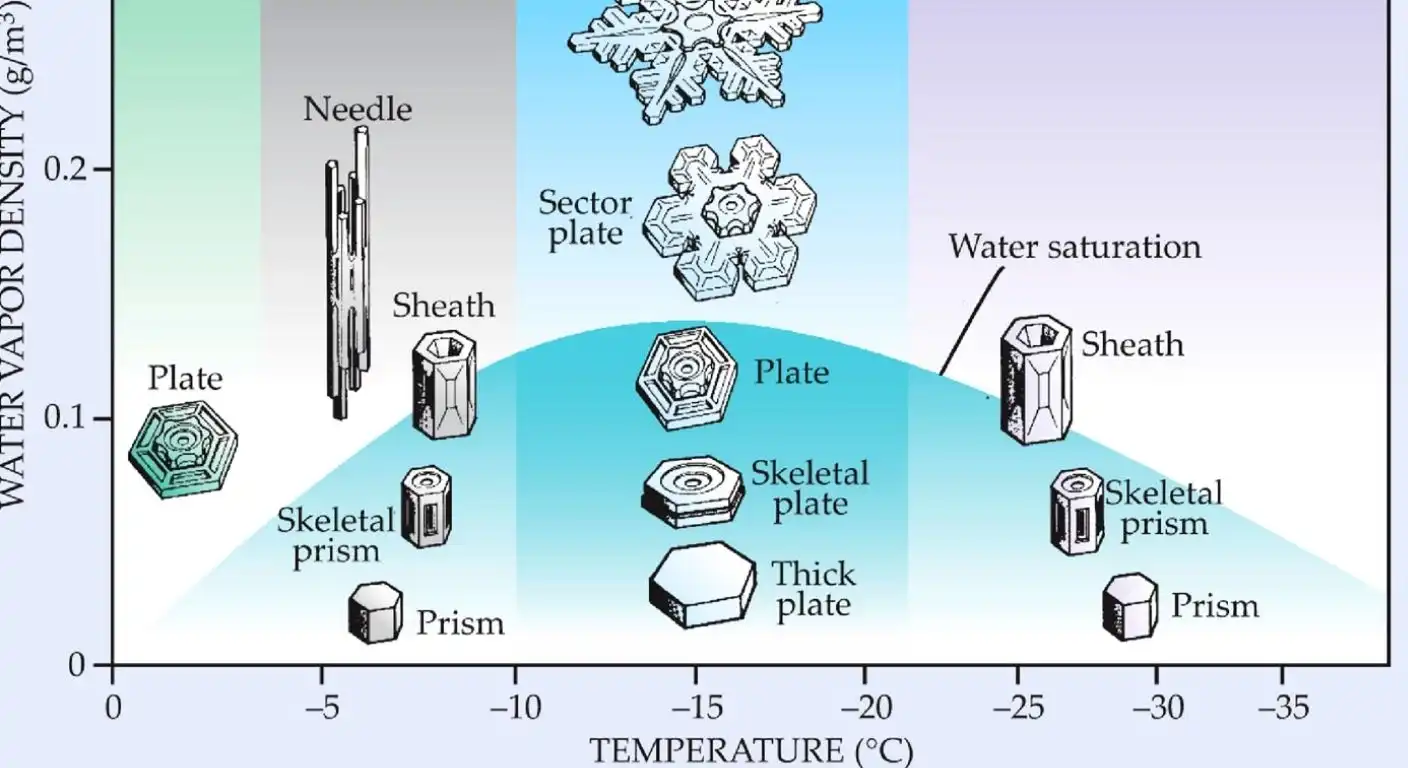
Why do snowflakes form in such a dazzling variety of intricate shapes? For over a century, scientists have been stumped by this question. Now, a researcher in Spain believes he has finally found the answer, and it has to do with a tiny, invisible layer of liquid water that exists on the surface of ice.
The idea of a “premelting film” on ice was first proposed by the famous physicist Michael Faraday, but it has since been a source of scientific debate. Some experiments have shown that it exists, while others have found no evidence of it.
In a new study, Luis MacDowell from the Universidad Complutense de Madrid used computer simulations to get a closer look at ice’s surface. He found that the thin liquid layer does indeed exist, but only under very specific conditions, right at the “triple point” where ice, liquid water, and water vapor can all coexist in perfect equilibrium.
MacDowell argues that the reason for all the past confusion is that it’s incredibly difficult to create these perfect equilibrium conditions in a lab. Even a tiny deviation can throw off the results and make the liquid layer disappear.
So, how does this explain the shape of snowflakes? MacDowell’s theory is that as the temperature and pressure in a snow cloud change, the thickness of this liquid film also changes. At certain points, the film undergoes a “surface phase transition,” like a tiny, two-dimensional version of water turning to ice. Each of these transitions causes a sudden change in the growth rate of the ice crystal’s different faces.
Because the face and the sides of the crystal grow at different speeds, a variety of shapes can emerge, from simple hexagonal prisms to the complex, branching dendrites we all know and love.
Source: The Journal of Chemical Physics (2026).