Key Points
- Ultrafast X-rays at SLAC revealed atomic changes in iron pentacarbonyl triggered by light.
- A novel theoretical method extracted atomic distances without complex simulations.
- Two carbon monoxide groups detached sequentially, with the second loss being less coordinated.
- A surprising “spectator” effect showed atomic vibrations spreading across the molecule.
In a groundbreaking study published in Nature Communications, scientists at the SLAC National Accelerator Laboratory have unveiled the ultrafast atomic motions that occur in iron pentacarbonyl after exposure to light. This offers new insights into how catalysts behave at the atomic level. This advancement, made possible using the Linac Coherent Light Source (LCLS), marks a significant leap in understanding the real-time dynamics of light-driven chemical reactions.
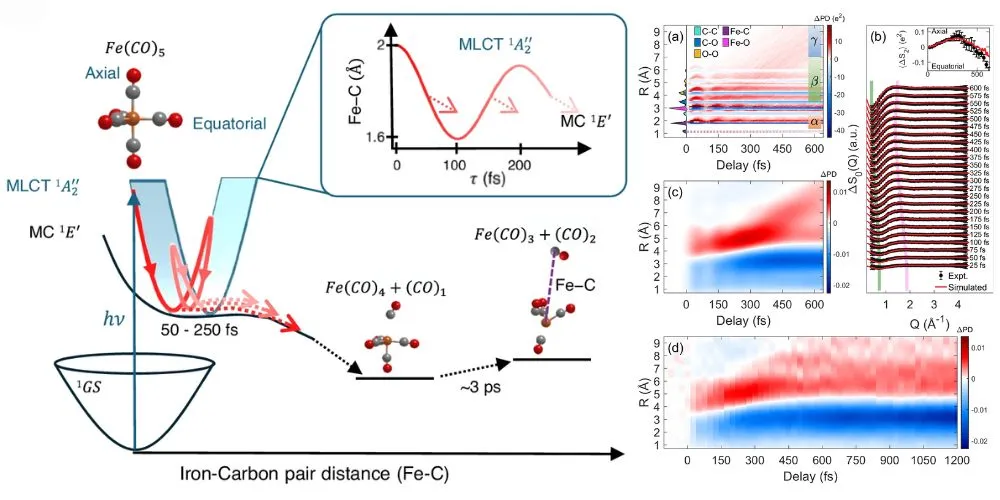
Iron pentacarbonyl is a molecule where a central iron atom is surrounded by five carbon monoxide (CO) groups. It sheds these CO groups upon exposure to light, enabling new molecules to bond in catalytic reactions. Although previous studies used spectroscopy to track energy flow, the exact atomic changes remained elusive.
To address this, researchers used ultrafast X-ray scattering at LCLS, capturing data on a femtosecond scale—millionths of a billionth of a second. These rapid X-ray pulses allowed them to observe how the molecule’s structure evolved following a flash of light.
However, limitations in detector size and data interpretation posed a challenge. Traditionally, such data is decoded by simulating potential molecular structures, a task made harder when metals like iron are involved. To overcome this, principal investigator Adi Natan developed a novel theoretical method for directly extracting atomic distances from scattering data without simulations.
This innovative approach revealed that iron pentacarbonyl loses one CO group after the light pulse, while the remaining CO groups rearrange. Later, a second CO group detaches with less structural coordination. Unexpectedly, researchers observed a “spectator” effect—vibrations initiated in one atomic pair spread through other atomic pairs, amplifying the original motion.
These findings demonstrate that tracking a single motion can unlock information about the entire molecule, even in more complex systems. By combining structural and spectroscopic data, scientists gain a comprehensive view of chemical processes, critical for designing more efficient catalysts in energy and industrial applications.
As Natan puts it, “Understanding how atoms move in real space and time brings us one step closer to controlling chemical reactions and designing new materials.”